Additional Homework Problems
CDP3-CB
For each of the following reactions and rate laws at low temperatures, suggest a rate law at high temperatures. The reactions are highly exothermic and therefore reversible at high temperatures.
(a) The reaction
A 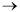 B
B
is irreversible at low temperatures and the rate law is
-rA = kCA
(a) The reaction
A + 2B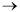 2D
2D
is irreversible at low temperatures and the rate law is
-rA = kCA1/2CB
(a) The catalytic reaction
A + B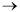 C + D
C + D
is irreversible at low temperatures and the rate law is

In each case, make sure that the rate laws at high temperatures are thermodynamically consistant at equilibriu, (cf. Appendix C).
[3rd Ed. P3-10]
 B
B 2D
2D C + D
C + D