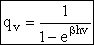
 (A12)
(A12)To show
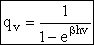
 (A12)
(A12)
Again we solve the wave equation for two molecules undergoing oscillation about an equilibrium position x = 0. The potential energy is shown below as a function of the displacement from the equilibrium position x = 0 (A5p402)
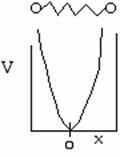
The uncertainty principle says that we cannot know exactly where the particle is located. Therefore zero frequency of vibration in the ground state, i.e. u = 0 is not an option (A5p402 and pA22). When vo is the frequency of vibration, the ground state energy is
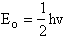 (V1)
(V1)
Harmonic oscillator (A5p402)
Spring Force 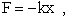 potential
energy from equilibrium position x = 0
potential
energy from equilibrium position x = 0

the solution is of the form for t=0 then x=0
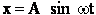
where
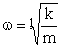
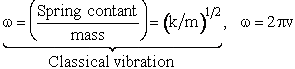
The potential energy is
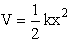 (V2)
(V2)
We now want to show
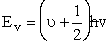 (V3)
(V3)
We now solve the wave equation:
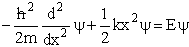 (V4)
(V4)
to find the allowable
energies, 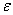 .
.
Let 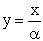 ,
,
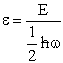 , where
, where 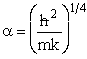 ,
,
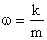 , and
, and 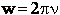
With these changes of variables Eqn. (A15) becomes
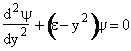 (V5)
(V5)
The solutions to this equation (A5 pA22, i.e., Appendix 8)will go to infinity unless
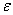 = 2
= 2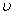 +1
+1
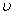 = 0, 1, 2, 3 . . .
= 0, 1, 2, 3 . . .
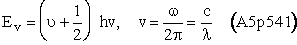 [c = speed of light]
[c = speed of light]
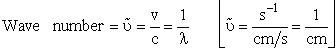
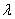 = wave length
= wave length
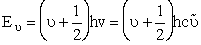 (V6)
(V6)
Measuring energy relative to the zero point vibration
frequency, i.e., 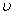 = 0
= 0
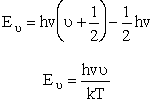
Substituting for 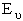 in
the partition function summation
in
the partition function summation 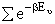

|
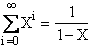
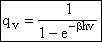 (V7)
(V7)
For 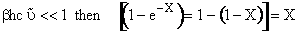 ,
we can make the approximation
,
we can make the approximation
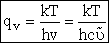 (V8)
(V8)
For m multiple frequencies of vibration
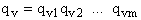
Order of Magnitude and Representative Values
For H2O
we have three vibrational frequencies with corresponding wave numbers, 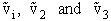 .
.
![]()
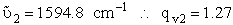
and
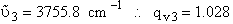
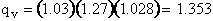
Return to Transition State Theory