The liquid-phase reaction 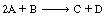 is carried out in a
semibatch reactor. The reactor volume is 1.2 m 3 . The
reactor initially contains 5 mol of B at a concentration of 0.015 kmol/m
3 . A at an aqueous concentration of 0.03 kmol/m 3
is fed to the reactor at a rate of 4 dm 3 /min. The reaction is
first order in A and half order in B with a specific reaction rate of
k = 6 (m 3 /kmol) 1/2 / min. The activation
energy is 35 kJ /mol. The feed rate to the reactor is discontinued when
the reactor contains 0.53 m 3 of fluid.
is carried out in a
semibatch reactor. The reactor volume is 1.2 m 3 . The
reactor initially contains 5 mol of B at a concentration of 0.015 kmol/m
3 . A at an aqueous concentration of 0.03 kmol/m 3
is fed to the reactor at a rate of 4 dm 3 /min. The reaction is
first order in A and half order in B with a specific reaction rate of
k = 6 (m 3 /kmol) 1/2 / min. The activation
energy is 35 kJ /mol. The feed rate to the reactor is discontinued when
the reactor contains 0.53 m 3 of fluid.
(a) Plot the conversion, volume, and concentration as a function of
time. Calculate the time necessary to achieve:
(b) 97% conversion of
A.
(c) 59% conversion of B.
(d) The reaction temperature is to be
increased from 25°C to 70°C and the reaction is to be carried out
isothermally. At this temperature the reaction is reversible with an
equilibrium constant of 10 (m 3 /kmol) 1/2 . Plot
the conversion of A and B and the equilibrium conversion of A as a
function of time.
(e) Repeat part (d) for the case when reactive
distillation is occurring. Study the effect of the evaporation rate on
conversion.
[2nd Ed. P4-27]