A bimolecular (elementary) second-order reaction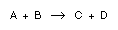 , takes place in a homogeneous liquid system. Reactants and
products are mutually soluble, and the volume change as a result of
reaction is negligible. Feed to a tubular (plug-flow) reactor that
operates essentially isothermally at 260°F consists of 210 lb/h of A and
260 lb/h of B. Total volume of the reactor is 5.33 ft 3 , and
with this feed rate, 50% of compound A in the feed is converted. It is
proposed that to increase conversion, a stirred reactor of 100-gal
capacity be installed in series with, and immediately upstream of, the
tubular reactor. If the stirred reactor operates at the same temperature,
estimate the conversion of A that can be expected in the revised system;
neglect the reverse reaction. Other available data include:
, takes place in a homogeneous liquid system. Reactants and
products are mutually soluble, and the volume change as a result of
reaction is negligible. Feed to a tubular (plug-flow) reactor that
operates essentially isothermally at 260°F consists of 210 lb/h of A and
260 lb/h of B. Total volume of the reactor is 5.33 ft 3 , and
with this feed rate, 50% of compound A in the feed is converted. It is
proposed that to increase conversion, a stirred reactor of 100-gal
capacity be installed in series with, and immediately upstream of, the
tubular reactor. If the stirred reactor operates at the same temperature,
estimate the conversion of A that can be expected in the revised system;
neglect the reverse reaction. Other available data include:

(Ans.: X = 0.68.) (California Professional Engineers Exam)
[2nd Ed. P4-11]