The irreversible liquid phase acid catalyzed reaction
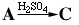
is carried out in a semibatch reactor containing H 2 SO
4 . A is fed at a constant rate of 10 mol/min. The volumetric
flow rate of liquid entering the semibatch reactor is 5 dm3
/min. The initial volume of a 3 M solution of H 2 SO
4 catalyst in the reactor is 100 dm3 (no A is
present initially). The specific reaction rate is 0.05 min-1 .
The reaction is first order in A and zero-order in catalyst concentration.
(a) Use POLYMATH or MATLAB to determine both the number of moles of A
in the tank and the concentration of A and of H 2 SO
4 as a function of time.
(b) Obtain an analytical solution
for the number of moles of A, NA , and the concentration
of A, CA , as a function of time. What is the concentration of
A after 30 min? How many moles of A will there be in a tank after long
times (i.e., 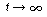 )? (Ans.: NA = 200 mol) Explain
why the number of moles remains virtually constant at long times.
)? (Ans.: NA = 200 mol) Explain
why the number of moles remains virtually constant at long times.
(c)
Rework part (a) assuming the reaction is first order in H 2 SO
4 with k = 0.02 dm 3 / mol min.
min.
(Hint: Do not try to use conversion in solving this problem!)