|
|
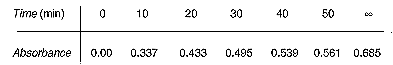
Penicillin G reacts with hydroxylamine (NO2OH) to form hydroxamic acid, which produces a colored complex with iron(III).
To determine the overall reaction order, equal concentrations of penicillin and NH2OH were mixed together in a 250-mL flask [J. Chem. Educ., 49,
539 (1972)]. Samples were withdrawn every 10 minutes and added to a solution containing
iron(III) chloride. With the aid of a colorimeter, the concentration of the colored
complex, and hence the concentration of hydroxamic acid, was obtained as a function
of time. The absorbance shown in the table below is directly proportional to the
hydroxamic acid concentration.
|
, and specific reaction rate, k.