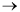 2CH4
2CH4The ethane hydrogenolysis over a commercial nickel catalyst was studied in a stirred tank solids reactor
H2 + C2H6 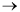 2CH4
2CH4
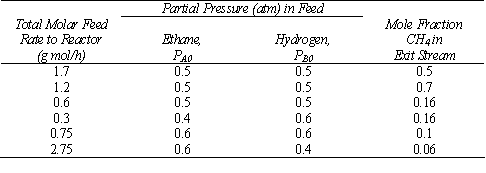
(a) Determine the rate law parameters from the data in the table below. There are four spnning baskets, each with 10 g of catalyst. Only hydrogen and ethane are fed to the reactor at 300 °C
(b) What experimental conditions would you suggest if you were to obtain more data?