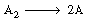

[1st Ed. P5-6]
| For the irreversible gas-phase dissociation of the dimer A2 | ||
|
|
||
| determine the CSTR volume necessary to achieve 80% conversion and produce 1000 g mol of A per hour. The feed stream consists of 60% A2 and 40% inerts at a pressure of 10 atm and a temperature of 40°C. The following data were obtained in the laboratory in a well-mixed constant-pressure batch reactor, which had an initial charge consisting of 85% A2 and 15% inerts. Process your data in terms of the measured variables (i.e., time and volume). | ||
|
[1st Ed. P5-6] |