The oxidation of propene (P) to acrolein (A) was carried out over a Mo-Pr-Bi catalyst [Ind. Eng. Chem. Res., 26, 1419 (1987)].
CH3CH=CH2+O2![]() CH2=CHCHO+H2O
CH2=CHCHO+H2O
It has been proposed to correlate the data using the power law model for the rate law [cf. Equation (5-2)].
racrolein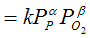
The reaction was carried out in a differential reactor with 0.5 g of catalyst at 623 K. From the data below, determine the reaction orders with respect to propene and oxygen
and oxygen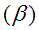 and the specific reaction rate, k.
and the specific reaction rate, k.
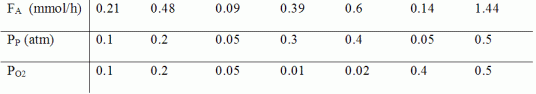
where
FA=exiting molar flow rate of acrolein, mmol/h
PP= entering partial pressure of propene, atm
PO2= entering partial pressure of oxygen, atm