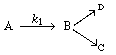
is carried out in a batch reactor in which there is pure A initially.
(a) Derive an equation for the concentration of A as a function
of time. If k 1 [2nd Ed. P9-12]
(b) Derive an equation that gives the concentration of B as a function of
time. If k 2 = 0.00 3 s -1 , k 3 = 0.002 s -1 , and C A0 = 0.2
g mol/dm 3 , what is the concentration
of B after 2 min?
(c) What is the concentration of C after 1 min? 2 min?
(d) Sketch the concentrations and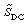 as functions of time. At
what time is the concentration of B at a maximum?
as functions of time. At
what time is the concentration of B at a maximum?
(e) If the series reaction is carried out in a CSTR, determine the reactor
volume that will maximize the production of B for a volumetric flow rate of 20 dm 3 / min.