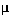 max = Maximum growth rate = 0.5 hr-1
max = Maximum growth rate = 0.5 hr-1Cells are growing at T=30°C in a continuous cell culturing system for cell growth kinetics study. Glucose is used as a carbon and energy source form. Cells are growing anaerobically and the fermentation product is acetic acid when 1 mole of glucose is fermented to produce 1 mole of acetic acid. Optimum cell growth rate can be obtained at pH=7. However, due to acetic acid production, pH goes down to around 4, at which cell growth is inhibited (product inhibition). In order to maintain the optimum pH, 0.1 N NaOH solution is added to the system.
Reactor Volume, V=10 dm3
NaOH solution inlet flow rate, Q = 0.025 dm3/hr
Inlet substrate (glucose) concentration, Sin = 10 g/ dm3
Ionization constant for acetic acid, Kacet = 1.75 x 10-5
Acetic acid inhibition kinetics = KP/(KP+CP)*, where KP = inhibition coefficient (*S. Aiba et al. Kinetics of Product Inhibition in Alcohol Fermentation, Biotech. Bioeng., 10:845-864,1968).
YX/S = mass of cells formed/mass of substrate consumed = 0.1
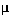 max = Maximum growth rate = 0.5 hr-1
max = Maximum growth rate = 0.5 hr-1
(a) For steady-state, solve the equations using Polymath to find CC, CS, and CP.
(b) If the dilution rate, D, in part (a) decreases 20% all of sudden, estimate what will happen to pH, CC, CS, and CP. Use Polymath.
Problem by Prof. D. S. Kim, University of Toledo