(b) Plot yj, wj as a function of j for degrees of polymerization of 5, 10, and 20. Plot P10 as a function of conversion of functional groups, p, for Mo= 1 mol/dm3
(c) Plot yj, and wj as a function of j for free radical termination by combination for p=0.8, p=0.9 and p=0.95.
(d) Determine the molecular weight distribution for the formation of polystyrene for an initiator concentration of 10-3 molar and a monomer concentration of 3 molar. What are
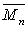 ,
, 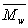 , and the polydispersity,
D, after 40 hrs? How would the
, and the polydispersity,
D, after 40 hrs? How would the 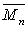 change if chain transfer were neglected?
change if chain transfer were neglected?(e) The polymerization of styrene is carried out in a batch reactor. Plot the mole fraction of polystyrene of chain length 10 as a function of time for an initial concentration of 3M and 0.01M of monomer and initiator respectively. The solvent concentration is 10 molar.