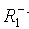 (i.e., R1), but
instead reacts at a finite rate with a specific reaction rate k0:
(i.e., R1), but
instead reacts at a finite rate with a specific reaction rate k0:Rework Example R7-3 from section 1 of the Professional Reference Shelf for the case when the initiator does not react
immediately with monomer to form the radical 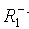 (i.e., R1), but
instead reacts at a finite rate with a specific reaction rate k0:
(i.e., R1), but
instead reacts at a finite rate with a specific reaction rate k0:
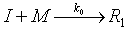
The rate law is
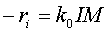
The initiator concentration at time at t=0 is I0.
(a)
Derive an equation for Rj as a function of 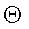 .
.
(b) For
M0= 3 mol/dm3, I0= 10-3
mol/dm3, k0= 0.1 dm3/mol*s, kp= 10
dm3/mol*s. Plot R8 and P8 as a function of real
time t.