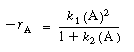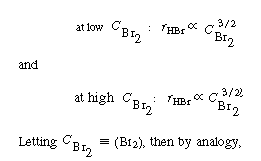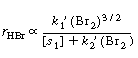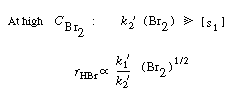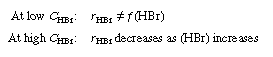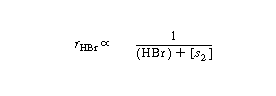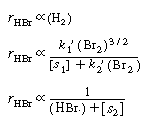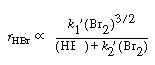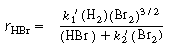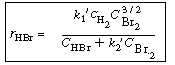| |
|
Develop a rate law for the HBr reaction that is consistent with the experimental
observations given above. |
|
| |
|
|
|
| |
|
Solution
It is customary in discussions of reactions of this sort to let the
species formula enclosed in parentheses represent its concentration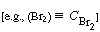 .
In the azomethane reaction, we saw that a rate law of the form .
In the azomethane reaction, we saw that a rate law of the form |
|
| |
|
|
|
| |
|
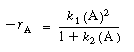
|
|
| |
|
|
|
| |
|
could account for the change in the apparent reaction order
when going from low to high concentration. |
|
| |
|
|
|
| |
|
For the HBr reaction |
|
| |
|
|
|
| |
|
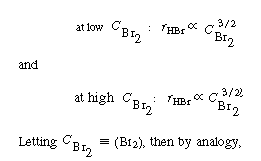
|
|
| |
|
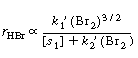
|
(CDE7-1.1) |
| |
|
|
|
| |
|
where [s1] represents
a reaction constant or the concentration of one of the species that will
be determined later |
|
| |
|
|
|
| |
|
and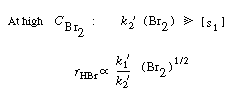
|
(CDE7-1.2) |
| |
|
|
|
| |
|
We see that the concentration dependence of bromine given
in Equation (CDE7-1.1) is consistent with experimental data.
Next consider the HBr dependence. |
|
| |
|
|
|
| |
|
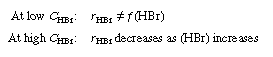
|
|
| |
|
|
|
| |
|
|
|
| |
|
One rate expression in which the concentration dependence
of HBr is consistent with experimental observation is |
|
| |
|
|
|
Going to the
extremes of high
and low
concentration
|
|
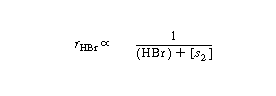 
|
(CDE7-1.3) |
| |
|
|
|
| |
|
Consequently, the concentration dependence of HBr denoted
by Equation (CDE7-1.31) is consistent with experimental observation.
This concentration dependence of the species H2,
Br2, and HBr in the rate expression are,
respectively, |
|
| |
|
|
|
| |
|
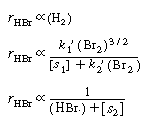
|
|
| |
|
|
|
| |
|
Comparing Equations (CDE7-1.1) and (CDE7-1.3) we see that
[s1] in Equation (CDE7-1.1) could be (HBr) and that [s2]
in Equation (CDE7-1.3) could be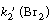 Therefore,
Equations (CDE7-1.1) and (CDE7-1.3) are combined to give Therefore,
Equations (CDE7-1.1) and (CDE7-1.3) are combined to give |
|
| |
|
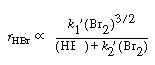
|
(CDE7-1.5) |
| |
|
|
|
| |
|
If we now incorporate dependence of the reaction rate on H2
[Equation (CDE7-1.4)] into Equation (CDE7-1.5), we arrive at the rate expression |
|
| |
|
|
|
| |
|
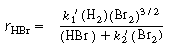
|
(CDE7-1.6) |
| |
|
|
|
| |
|
or, identically, |
|
| |
|
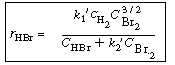
|
(CDE7-1.7) |
| |
|
which is consistent with all experimental observations. |
|