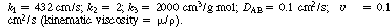
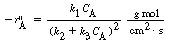
(a) What is the lowest feed concentration, C A0 , one can have and still have the possibility of multiple steady states?
(b) Derive an equation relating the wire diameter D to the velocity U, which divides a plot of U vs. D into two regions, one in which a multiple steady state (MSS) is possible and one in which a MSS is not possible.