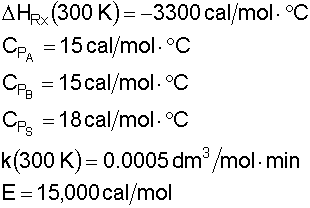
is carried out adiabatically in a CSTR.
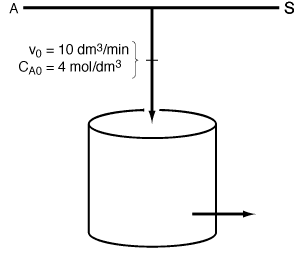
Chapter 8, Figure CDE8-2.1
The reaction is second order in A. The feed, which is equimolar in a solvent (which contains the catalyst) and A, enters the reactor at a total volumetric flow rate of 10 dm 3 /min with the concentration of A being 4M. The entering temperature is 300 K.
(a) What CSTR reactor volume is necessary to achieve 80% conversion?
(b) What conversion can be achieved in a 1000 dm 3 CSTR? What is the new exit temperature?
(c) How would your answers to part (b) change, if the entering
temperature of the feed were 280 K?
Additional Information: