Additional Homework Problems
P8-29B
Exothermic reversible reaction with a variable coolant temperature. The elementary reaction
A + B 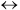 2 C
2 C
is carried out in a PBR that has a heat exchanger surrounding the reactor. The feed is equal molar in A and
b with FA0 = 5 mol/s. The coolant surrounding the PBR flow in the same direction as the reactants. The feed enters at a temperature of 330 K. Plot X, Xe and T as a function of catalyst weight up to W = 4500 kg, for the base case given below.
Additional Information (base case)
E = 25 kcal/mol
CPA = CPB = CPC = 20 cal/mol/K
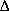 HRX = -20 kcal/mol
HRX = -20 kcal/mol
CPI = 40 cal/mol/K
k = 0.004 dm6/(kg s) at 310 K
Ua/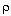 B = 0.5 cal/(kg s K)
B = 0.5 cal/(kg s K)
KC = 1000 at 303 K
Ta = 320 K
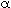 = 0.002 /kg
= 0.002 /kg
mC = 1000 g/s
Fao = 5 mol/s
CPmc = 18 cal/g/K
CTo = 0.3 mol/dm3
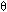 I = 1
I = 1
Vary the following parameters and write a paragraph describing the trends you find for each parameter variation and why they work the way they do. Use the base case for parameters not varied.
- FA0 1
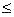 FA0
FA0 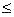 8 mol/s
8 mol/s
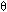 I 0.5
I 0.5 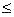
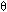 I
I 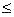 4
4
- Ua/
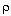 B 0.1
B 0.1 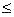 Ua/
Ua/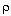 B
B 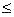 0.8 cal/(kg s K)
0.8 cal/(kg s K)
- T0 310 K
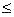 T0
T0 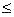 350 K
350 K
- Ta 300 K
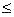 Ta
Ta 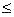 340 K
340 K
- mC 1
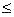 mC
mC 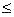 1000 g/s [ T0 = 350 , Ua/
1000 g/s [ T0 = 350 , Ua/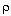 B = 0.5, Ta = 320, FA0 = 5,
B = 0.5, Ta = 320, FA0 = 5, 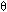 I = 1]
I = 1]
- Determine the conversion in a 5000 kg fluidized CSTR where UA/
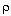 B = 500 cal/(kg s K)
B = 500 cal/(kg s K)
- If the reaction were endothermic with KC = 0.01 at 303 and
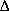 HRX = +20 kcal/mol
HRX = +20 kcal/mol
[3rd Ed. P8-15]
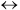 2 C
2 C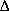 HRX = -20 kcal/mol
HRX = -20 kcal/mol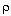 B = 0.5 cal/(kg s K)
B = 0.5 cal/(kg s K)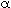 = 0.002 /kg
= 0.002 /kg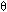 I = 1
I = 1
 FA0
FA0