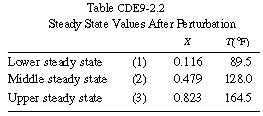
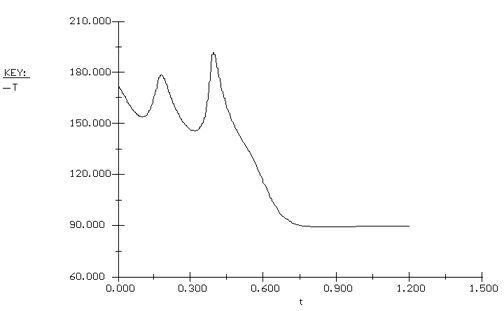
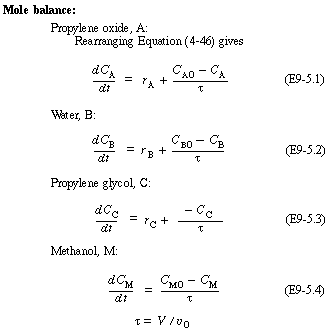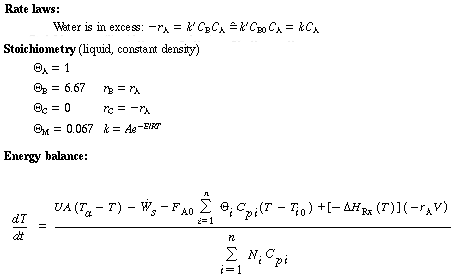Propylene glycol is produced in a CSTR in which precautions have been taken to prevent product loss. Propylene oxide (A) is fed to a 413-gal reactor at a rate of 300 lb mol/h at a temperature of 75°F. Water (B) is fed at a rate of 2000 lb mol/h and methanol at a rate of 20 lb mol/h. Determine what happens to a CSTR operating at 172°F and a conversion of 86.4% when there is a drop in feed temperature from 75°F to 68°F.
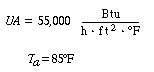
The property values are the same as those given in Example 8-4.
Solution
We will first look at the steady-state conditions before the upset occurred. Recall Text Equation (E8-8.5), obtained from the mole balance,
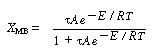
and Equation (E8-8.6) obtained from the energy balance,
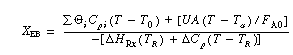
Neglecting![]() and
substituting the appropriate values, we have
and
substituting the appropriate values, we have
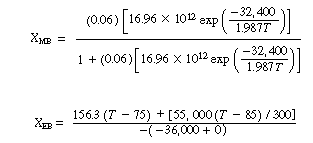
(CDE9-2.1)
(CDE9-2.2)
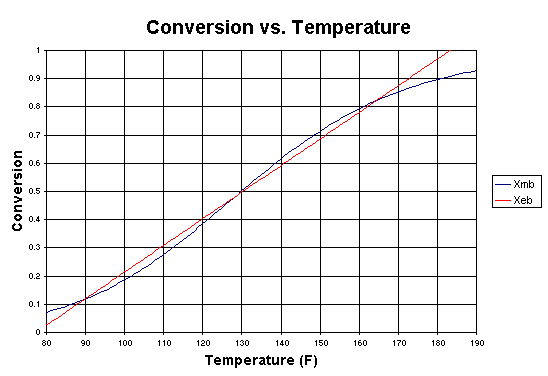
If we were to plot XEB and XB as a function of temperature, we would we see that there are three steady-state conditions for this set of parameter values (Table CDE9-2.1). Before the drop in feed temperature occurred we operated at the upper steady state (T = 172°F, X = 0.864). The ignition temperature of the feed is 78°F, and the extinction temperature is 61°F (review Figure 8-19).