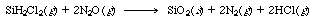
| Silicon dioxide is formed by chemical vapor deposition from dichlorosilane (DCS) and nitrous oxide [Proceedings of the Third World Congress of Chemical Engineering, Tokyo, p. 290 (1986)]. | ||
|
|
||
| At 900°C the rate of deposition is | ||
|
|
|
|
| The deposition rate is independent of HCl and nitrogen. At 900°C | ||
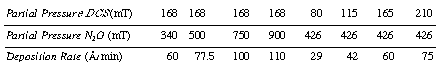
|
| (a) Can the reaction order with respect N 2
O and DCS be explained by some other means than powers that the author used?
If so, formulate a new rate law and evaluate the parameters. (b) Is the following mechanism consistent with the rate law? |
||
|
|
|
In this mechanism we assume that the last step is extremely rapid
in that as soon as SiO (c) The wafers are 0.125 m in diameter and set upright along the length of the reactor. Silicon dioxide is deposited uniformly on both sides of the wafers. The reactor diameter is 250 mm and the wafers are spaced 20 mm apart. This arrangement corresponds to a deposition surface area of 250 m2 per cubic meter of reactor volume. Assume that the gas phase behaves as a plug-flow reactor at steady state. Develop the equations for the axial deposition profile. Specifically, determine the thickness of the deposited film at 900°C on the 1st, 50th, and 110th wafers in the reactor after 30 min. Dichlorosilane is fed at a partial pressure of 157 mT and a rate of 0.00368 mol/ min, while nitrous oxide is fed at a partial pressure of 447 mT and a rate of 0.013 mol/ min. [2nd Ed. P6-13] |