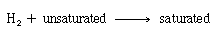
The reaction in the pellet is first-order in both hydrogen and
the organic. Hydrogen and nitrogen are fed in equimolar portions at a total pressure
of 2 atm. The reactor diameter is to be 1.0 m. It is proposed to operate at a superficial
liquid mass velocity of 5 kg/m 2 s and a total gas flow rate of 36 mol/s. As a first approximation assume
that the concentration of organic is constant and the pseudo-first-order specific
reaction rate is 2.5 x 10 -5
m 3 /kg cat.
s and a total gas flow rate of 36 mol/s. As a first approximation assume
that the concentration of organic is constant and the pseudo-first-order specific
reaction rate is 2.5 x 10 -5
m 3 /kg cat. s
at 400 K.
s
at 400 K.
(a) Calculate the catalyst weight necessary to achieve 55%
conversion of the hydrogen.
(b) If 55% conversion could not be obtained for the operating conditions specified,
what should the operating conditions be to obtain this conversion?
(c) For each transport step, determine its fraction of the total resistance to
mass transport and reaction.
(d) The bed is now to be operated under conditions of complete saturation
of the hydrogen throughout the bed. Calculate the weight of catalyst necessary to
achieve 95% conversion of the organic. To achieve this conversion the pressure is
increased to 100 atm and the feed is pure H 2. The particle size is reduced by a factor of 4.
(e) Assuming an axial dispersion coefficient of 0.38 cm2 /s, determine if the plug-flow model was
a good assumption.
Liquid viscosity: 3.1 cP = 0.0031 kg/m
 s
sLiquid density: 700 kg/m 3
Hydrogen liquid diffusivity in oil: 7.0 x 10 -9 m 2 /s
Organic diffusivity in organic product: 2.5 x 10 -9 m 2 /s
Molecular weight of organic: 256 Daltons
Hydrogen solubility: 0.004 kmol/m 3
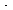 atm
atmPellet porosity: 0.45
Pellet density: 1600 kg/m 3
Bed porosity: 0.4
Superficial gas velocity (kg/m 2
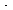 s): 0.75 0.5 0.1
s): 0.75 0.5 0.1Pressure drop (kPa/m): 70 50 20
[From AIChE J., 29, 1 (1983)]