![xi]](../../images/xi.gif) p = 0.6,
p = 0.6,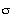 = 0.8.
= 0.8.
(b) Determine the conversion of ethanol.
(c) Assuming an axial dispersion coefficient of 0.02 cm 2/s, determine if the plug-flow model was a good assumption.
The oxidation of ethanol is to be carried out in a trickle bed reactor.
The reaction is first-order with respect to oxygen and zero-order with respect to
ethanol. The reactor is 30 cm in diameter and 6 m long and the bed porosity is 40%.
The Pd/Al 2 O catalyst pellets
are 0.5 cm in diameter and have a density of 1800 kg/m 3 . The diffusivity of oxygen in ethanol is 4 x 10 -10 m2 /s and the equilibrium solubility is 4
x 10 -2 kmol/m3 at reaction conditions. The superficial
gas velocity is 10 cm/s and the corresponding pressure gradient is 30 kPa/m. The
superficial liquid-phase velocity is 0.25 cm/s. The specific reaction rate is 0.03
s -1 . Tortuosity = 3.9,![xi]](../../images/xi.gif) p = 0.6, p = 0.6,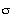 = 0.8. = 0.8.
|
||
| (a) Calculate the percent of the total resistance for each
transport step. (b) Determine the conversion of ethanol. (c) Assuming an axial dispersion coefficient of 0.02 cm 2/s, determine if the plug-flow model was a good assumption. |
[2nd Ed. P12-9]