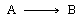
| The irreversible catalytic isomerization | ||
|
|
||
| is to be carried out in fluidized bed reactor. It is desired to process 8200 liters per hour of feed stock (at 100°C) containing 50% A and 50% inerts and to achieve 90% conversion. The reaction is first order in A. The feed is considered to have the same physical--properties as air. | ||

|
| (a) What is the minimum fluidization velocity? (b) What is the bed porosity at the minimum fluidization velocity u m ? (c) If you are to operate at 5 times u m , what should your bed diameter be? (d) What is the entrainment velocity for this particle size? (e) What is the minimum bubble diameter? (f) What is the maximum bubble diameter? (g) What is the bubble velocity? (h) What is the cloud diameter? (i) What is the fraction of the bed occupied by the bubble? (j) What is the fraction of bed occupied by the wake? (k) What is the velocity of the solids flowing downward? (l) What is the velocity of rise of the gas in the emulsion phase? (m) Use the balances on emulsion and cloud phases to eliminate CAC and C Ae in order to show that the balance on the bubble phase can be written as |
||
|
|
||
|
(n) Calculate Kbc (p) Calculate
|