
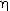 :
:
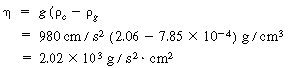
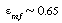
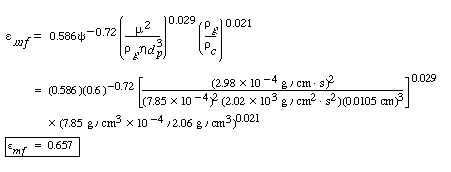
(CD12-29)
| Massimilla and Johnstone 25 studied the catalytic oxidation of ammonia in a fluidized-bed reactor. Under their experimental conditions, the reaction was first order, dependent only on the ammonia concentration, and without a significant change in volumetric flow rate. In one of their runs, 4 kg of catalyst was used with a gas flow rate of 818 cm 3 /s at reaction conditions. A conversion of 22% of the entering ammonia was obtained. Predict this conversion using the Kunii-Levenspiel model. | ||||
| Other data: | ||||

|
||||
| Solution | ||||
| A. Mechanical characteristics of bed | ||||
Step 1. Gravitation term, 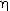 : :
|
||||
|
|
||||
| Step 2. Porosity of bed at minimum fluidization: | ||||
|
|
|
(CD12-29) |
||
| Step 3. Gas velocity at minimum fluidization: | ||||
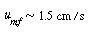
|
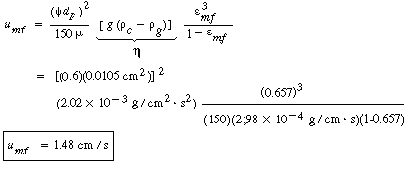
|
(CD12-25) |
| Step 4. Entering gas velocity: | |||
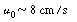
|
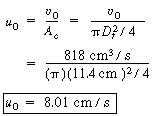
|
||
| Step 5. Is u 0 within a reasonable operating range? Check u t . | |||
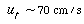
|
|
(CD12-33) |
|
| Are the N Re in the proper range for use of Equations (CD12-25) and (CD12-33)? | |||
|
|
|||
Thus u 0
is 5.4 times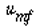 , and well below u t . , and well below u t .
|
|||
| Step 6. Bubble sizes, d b 0 , d bm , and d b : | |||
|
|
|
(CD12-38) (CD12-39) |
| Step 7. Bubble sizes, d b 0 , d bm , and d b : The unexpanded bed height is 38.9 cm. The expanded bed height will probably be 40 to 50% greater, say about 60 cm. We will therefore assume that the average bubble size will be taken as the one calculated for h /2 = 30 cm. | ||||
| Step 8. Average bubble diameter: | ||||
|
|
|
|||
| Step 9. Rise velocity of single bubble: | ||||
|
|
(CD12-35) | |||
| Step 10. Rise velocity of a bubble when many bubbles are present: | ||||
|
|
|
(CD12-36) | ||
From Figure CD12-6 for glass spheres with d p
= 0.105 mm,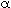 = 0.4. = 0.4.
|
||||
| Step 11: Fraction of bed in bubble phase: | ||||
|
|
|
(CD12-46) |
||
| Step 12. Bed height: | ||||
|
|
||||
|
Since the estimated bed height of 60 cm is sufficiently close to the calculated value of 63.2 cm, one can proceed further in the calculations without making a new estimate of h. | |||
| B. Mass transfer and reaction parameters | ||||
| Step 1. Bubble-cloud mass transfer coefficient: | ||||
|
|
(CD12-53) |
| Step 2. Cloud-emulsion mass-transfer coefficient., | ||||
|
|
|
(CD12-55) | ||
| Step 3. Volume of catalysts in the bubble per volume of bubble: | ||||
|
|
||||
| Step 4. Volume of catalyst in clouds and wakes/ cm 3 of bubbles: | ||||
|
|
|
(CD12-63) |
||
| Step 5. Volume of catalyst in emulsion/cm 3 of bubbles: | ||||
|
|
|
(CD12-64) |
||
| rearranging Equation (CD12-75). | ||||
| Step 6. Calculate K R and X from Equation (CD12-27): | ||||
|
|
||||
| where | ||||
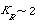
|
|
(CD12-72) |
||
| Solving for X gives | ||||
|
|
||||
| This is close to the observed value of 22% conversion. |