 C
CA decaying catalyst is fed to a fluidized CSTR at a rate of 20 kg/s. The 1-m3 CSTR contains 200 kg of catalyst. The catalyst decay follows second-order kinetics. The elementary gas-phase reaction,
A + B 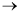 C
C
takes place in the reactor. A and B are fed in stoichiometric proportions. The concentration of A is 0.04 mol/dm3 and the total entering volumetric flow rate is 10 dm3/s.
(a) What is the mean activity of the catalyst exiting the reactor?
(b) What is the mean activity of the catalyst inside the reactor?
(c) What conversion of A is predicted from the balance,
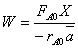
(d) What conversion of A is predicted from the segregation model?
(e) What catalyst weight is necessary to achieve 75% conversion?
(f) What conversion would be achieved if the catalyst feed rate to the reactor were increased by a factor of 5?
(g) How would your answers to parts (c), (d), and (e) change if the decay were first-order?