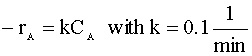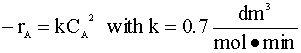Chapter 1: Mole Balances
Additional Homework Problems
CDP1-AA
A 200 dm3 constant-volume batch reactor is pressurized to 20 atm with a mixture of 60% A and 40% inert. The gas-phase reaction is carried out isothermally at 227°C.  V = 200-dm3 P = 20 atm T = 227°C
|