Chapter 2: Conversion and Reactor Sizing
Additional Homework Problems
CDP2-AB
For the irreversible gas-phase reaction:
![]()
the following correlation was determined from laboratory data (the initial concentration of A is 0.2 g mol/L):
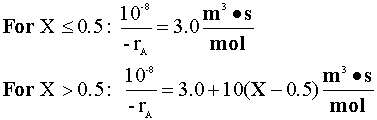
The volumetric flow rate is 5 m3/s.
- Over what range of conversions are the plug-flow reactor and
CSTR volumes identical?
- What conversion will be achieved in a CSTR that has a volume
of 90 L?
- What plug-flow reactor volume is necessary to achieve
70% conversion?
- What CSTR reactor volume is required if effluent from the
plug-flow reactor in part (c) is fed to a CSTR to raise the
conversion to 90%?
- If the reaction is carried out in a constant-pressure
batch reactor in which pure A is fed to the reactor, what
length of time is necessary to achieve 40 % conversion?
- Plot the rate of reaction and conversion as a function of
PFR volume.
- Critique the answers to this problem.