Chapter 3: Rate Laws
Additional Homework Problems
CDP3-A
For families of reactions, the Polanyi-Semenov equation can be used to estimate activation energies from the heats of reaction, ![]() according to the equation,
according to the equation,
E = C - ![]() (
(![]() )
)
where ![]() and C are constants. For expthermic reactions,
and C are constants. For expthermic reactions, ![]() = -0.25 and C = 48 kJ/mol, while for endothermic reactions,
= -0.25 and C = 48 kJ/mol, while for endothermic reactions, ![]() = -0.75 and C = 48kJ/mol. However, these values may vary somewhat from reaction family to reaction family [K.J. Laidler, Theories of Chemical Reaction Rates (New York, R. E. Krieger, 1979), p. 38]. (Also see Appendix J)
= -0.75 and C = 48kJ/mol. However, these values may vary somewhat from reaction family to reaction family [K.J. Laidler, Theories of Chemical Reaction Rates (New York, R. E. Krieger, 1979), p. 38]. (Also see Appendix J)
(a)Why is this a reasonable correlation?
Consider the following family of reactions:
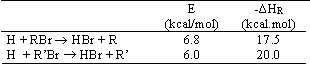
(b)Estimate the activation energy for the reaction
![]()
which has an exothermic heat of reaction of 6 kcal/mol (i.e., ![]() = -6 kcal/mol)
= -6 kcal/mol)