Chapter 3: Rate Laws
More on Activation Energy
The activation energy can be thought of as a barrier to the reaction. One way to view the barrier to a reaction is through the reaction coordinates. These coordinates denote the energy of the system as a function of progress along the reaction path. For the reaction
![]()
the reaction coordinate is
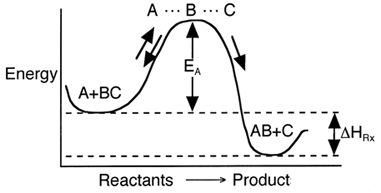
Progress along reaction path
We see that for the reaction to occur, the reactants must overcome an energy barrier or activation energy EA.