Chapter 3: Rate Laws
Example: Gas Phase Catalytic Reactions
When studying gas phase catalytic reactions the rate law is developed in terms of partial pressure,
e.g., ![]()
To rewrite the rate law, just use ideal gas law to relate to concentrations CA and CB
![]() ,
,![]()
and then write concentration in terms of conversion.
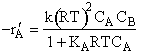
![]()