Chapter 4: Stoichiometry
Additional Homework Problems
CDP4-KB
The following elementary gas phase reaction takes place in a constant-pressure isothermal vessel (1 atm, 25![]() ):
):
CH4(g) + 2Cl2(g) ![]() CH2Cl2(g,l) + 2HCl(g)
CH2Cl2(g,l) + 2HCl(g)
Taking chlorine as your basis of calculation:
(a) Set up the stoichiometric table, assuming that the feed is in stoichiometric proportions and comprised only of reactants.
(b) Evaluate 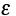 .
.
(c) Evaluate -rAin terms of X, the conversion of chlorine, the specific reaction rate, and the initial concentration of chlorine.
(d)What is the concentration of chlorine at 60% conversion."
(e)What is the rate of reaction when X = 0.6?
(f) If the frequency factor is 2*1012 liters2/s*g-mol2, calculate the activation energy.
(g)What is the specific reaction rate at 100 ![]() ? (Note: One of the products is a liquid with a vapor pressure of 400 mmHg = 53 kPa at 25
? (Note: One of the products is a liquid with a vapor pressure of 400 mmHg = 53 kPa at 25 ![]() )
)
Additional Data:
k = 0.2dm6/s*g-mol2 at 25(estimate)
R = 1.987 cal/g-mol*K = 0.082 dm3*atm/g-mol*K