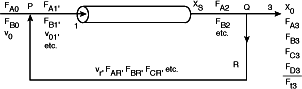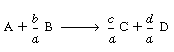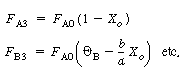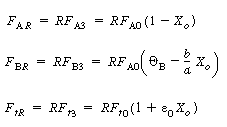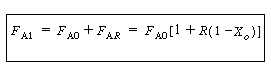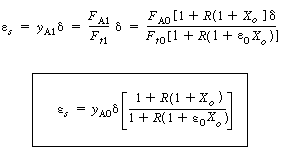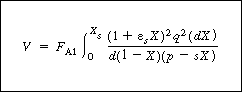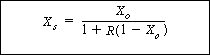Chapter 5: Isothermal Reactor Design: Conversion
Professional Reference Shelf
R5.2 Recycle Reactors
| Recycle reactors are used when the reaction is autocatalytic, or when it is necessary to maintain nearly isothermal operation of the reactor or to promote a certain selectivity. They are also used extensively in biochemical operations. To design recycle reactors, one simply follows the procedure developed in this chapter and then adds a little additional bookkeeping. A schematic diagram of the recycle reactor is shown below. | |||
|
|
|||
| The recycled stream is drawn off at point Q and merged with the fresh feed at Point P. We shall define the recycle parameter R as the moles recycled per mole of product removed at point Q. | |||
|
|
|||
|
Two conversions: |
Two conversions are usually associated with recycle reactors: the overall conversion, X0, and the conversion per pass, Xs : | ||
|
|
(CD5-88) (CD5-89) |
||
|
The only new twist in calculating reactor volumes or conversions for a recycle reactor is a mole balance at the stream intersections (points P and Q) to properly express the species concentrations as a function of conversion. Consider the gas-phase reaction
occuring in our reactor. Let X be the conversion of A in the reactor per mole of A fed to the reactor. The design equation is |
|||
|
|
|||
| Then: | |||
| Design equation: | |||
|
|
|||
| Rate law: | |||
|
|
|||
| with |
|||
| Stoichiometry: 1. From the definition from the overall conversion, we can define F A3 and F B3 leaving the system, |
|||
|
|
(CD5-90) (CD5-91) |
||
| From the definition for conversion per pass, we can define F A2 and F B3 leaving the reactor, | |||
|
|
(CD5-92) (CD5-93) |
||
| 2. From the definition for the recycle parameter, R, we can define F AR and F BR and the total molar flow rate in the recycle stream, F tR | |||
|
|
(CD5-94) (CD5-95) (CD5-96) |
||
| where | |||
|
|
|||
| 3. From the balance on the stream intersections, we have | |||
|
|
(CD5-97) |
|
Relating the molar flow rates in the various streams |
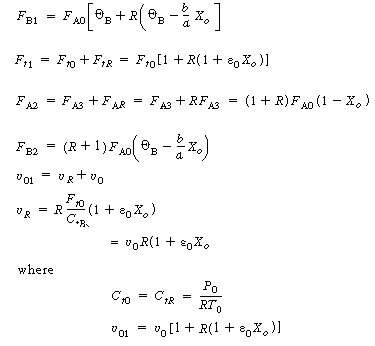 |
|
| The volumetric flow rate in the reactor, , is related to the volumetric
flow rate entering the reactor |
|||||
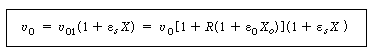 |
(CD5-107) | ||||
| where X is the number of moles of A reacted per mole of A entering
the reactor, and |
|||||
|
|
(CD5-108) |
||||
| The molar flow rate of A within the reactor is | |||||
|
|
(CD5-109) | ||||
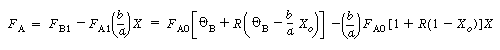 (CD5-110)
(CD5-110)
|
|
(CD5-111) | ||||
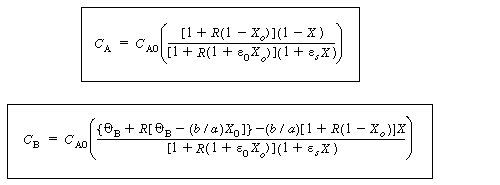 |
(CD5-112) (CD5-113) |
||||
| These equations for concentration are substituted into the rate law, which is in turn substituted into the design equation and integrated. For a first-order reaction in A and in B, | |||||
|
|
(CD5-114) | ||||
|
Recycle reactor volume |
|
(CD5-115) | |||
| where | |||||
|
|
|||||
| The relationship between the overall conversion and the conversion per pass can be found by equating F A2 from Equations (CD5-107) and (CD5-106): | |||||
|
|
|||||
| Then using Equation (CD5-97) and simplifying, we have | |||||
|
|
(CD5-116) | ||||