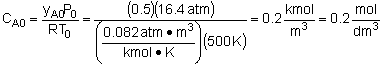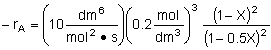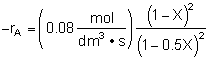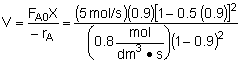Chapter 5: Isothermal Reactor Design: Conversion
Elementary gas phase reaction in different reactor types.
CSTR
The elementary gas phase reaction
![]()
takes place in a CSTR at constant temperature (500 K) and constant pressure (16.4 atm). The feed is equal molar in A and B.
|
Mole Balance |
|
|
Rate Law |
|
|
Stoichiometry |
gas phase, isothermal (T = T0), no pressure drop (P = P0) |
|
|
|
|
|
|
Why do you suppose CB is a constant, when B is being consumed? |
|
|
|
|
|
Combine |
|
|
|
|
|
|
|
|
Evaluate |
|
|
|
PFR and Batch Reactors
Elementary Gas Phase Reaction:
![]()
PFR
| Mole Balance | 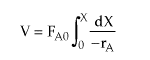 |
| Rate Law | |
| Stoichiometry | gas phase, isothermal (T = T0), no pressure drop (P = P0), CAo=CBo (Q=1), v=vo(1+eX) |
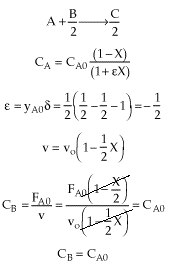 | |
| Combine | 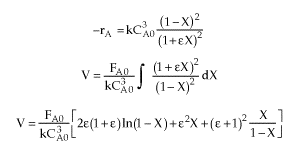 |
| Parameter Evaluation | CAo=0.2, v=vo=25 dm3/s, k=10 dm6/mol2 s, e=-0.5, X=0.9 |
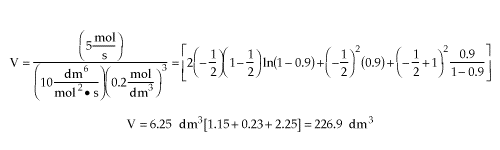 | |
| V=227 dm3 |
Batch Reactor Constant Volume, V=Voand the pressure changes.
| Mole Balance | |
| Rate Law | |
| Stoichiometry | |
| Combine | |
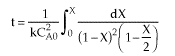 | |
| Parameter Evaluation | CAo=0.2, k=10 dm6/mol2 s, |
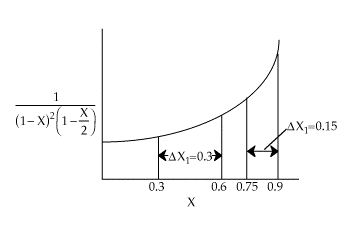 | |
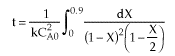 | |
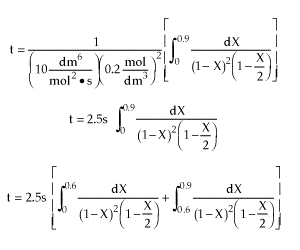 | |
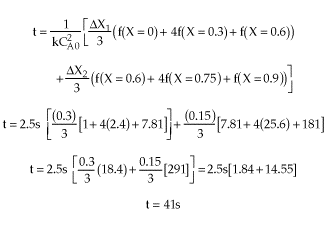 |
|
|
|