Chapter 6: Isothermal Reactor Design: Molar Flow Rates
Additional Homework Problems
CDP6-GB
Radial-flow reactors can be used to good advantage for exothermic reactions with high heats of reaction. The high radial velocities at the entrance to the reactor are useful in reducing hot spots within the reactor. As fluid moves out into the reactor, the velocity, U, varies inversely with r:
![]()
where U 0 is the velocity (m/s) at the inlet radius, R irreversible, gas-phase reaction R 0. Consider the elementary, irreversible, gas-phase reaction
![]()
carried out in a radial-flow reactor similar to the one shown in Figure CDP6-N. Derive an equation for conversion as a function of radius.
k1
A+B → C+D
(a) Derive an equation for conversion as a function of radius
for isothermal operation neglecting pressure drop.
(b) Plot X as a function of r for the case when the pressure drop is
significant with a 5 0.07 kg -1 .
(c) Vary the reaction and reactor parameter values and write a paragraph describing
your findings. What parameter effects the results the most?
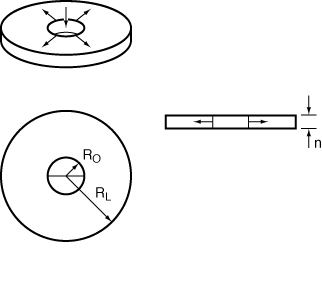
Figure CDP6-N
Suggested parameter values:
![]() , F A0 = 10
mol/min, C A0
= 0.1 mol/dm 3 ,
, F A0 = 10
mol/min, C A0
= 0.1 mol/dm 3 ,
h = 0.4 dm, R 0R 1
Bulk density of catalyst
2000 g/dm 3
[2nd Ed. P4-31]