Chapter 6: Isothermal Reactor Design: Molar Flow Rates
Additional Homework Problems
CDP6-MB
The elementary gas-phase isomerization reaction
A → B
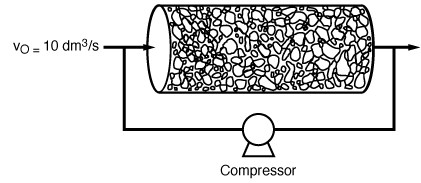
is carried out in a packed-bed recycle reactor. The recycle
ratio is 5 mol recycled per mole taken off in the exit stream. For a volumetric flow
rate of 10 dm 3 /s through the reactor (Figure CDP6-L),
the corresponding pressure gradient (assumed constant) in the reactor is 0.0025 atm/
m. The flow in the reactor is turbulent. What overall conversion can be achieved
in a reactor that is 10 m in length and 0.02 m 2
in cross-sectional area? Figure CDP6-L
Additional information:
C A0 =0.01 mol /dm 3
(P 0 = 2 atm). The volumetric
flow rate of fresh reactant to the reactor is v0
= 10 dm 3 /s. The specific reaction
rate is k = 0.25 s -1 .
[1st Ed. P4-22]