Chapter 7: Collection and Analysis of Rate Data
Additional Homework Problems
CDP7-BB
Oxygenating Blood
When arterial blood enters a tissue capillary, it exchanges oxygen and carbon dioxide with its environment, as shown in this diagram.
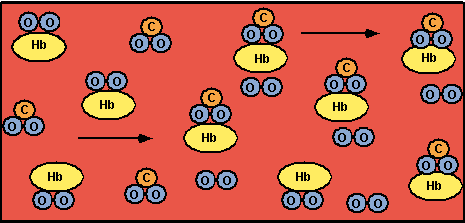
The kinetics of this deoxygenation of hemoglobin in blood was studied with the aid of a tubular reactor by Nakamura and Staub [J. Physiol., 173, 161 (1967)].
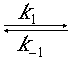 Hb +
O2
Hb +
O2Although this is a reversible reaction, measurements were made in the initial phases of the decomposition so that the reverse reaction could be neglected. Consider a system similar to the one used by Nakamura and Staub: the solution enters a tubular reactor (0.158 cm in diameter) that has oxygen electrodes placed at 5-cm intervals down the tube. The solution flow rate into the reactor is 19.6 cm3/s.
| Electrode Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Percent Decomposition of HbO2 | 0.00 | 1.93 | 3.82 | 5.68 | 7.48 | 9.25 | 11.00 |
Using the method of differential analysis of rate data, determine the reaction order and the forward specific reaction rate constant k for the deoxygenation of hemoglobin.
[Hint: Estimate the inlet concentration of HbO2 by using typical
values of hemoglobin concentration in adult human blood, and by assuming that
hemoglobin is saturated with oxygen]
Solution