Chapter 8: Multiple Reactions
Additional Homework Problems
CDGA 8-1
(Methanol synthesis) A new catalyst has been proposed for the synthesis of methanol from carbon monoxide and hydrogen gas. This catalyst is reasonably active between temperatures of 330 K to about 430 K. The isothermal reactions involved in the synthesis include:
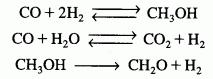
the reactions are elementary and take place in the gas phase. The reaction is to be carried out isothermally and, as a first approximation, pressure drop will be neglected. The feed consists of 7/15 hydrogen gas, 1/5 carbon monoxide, 1/5 carbon dioxide, and 2/15 steam. The total molar flow rate is 300 mol/s. The entering pressure may be varied between 1 atm and 160 atm, and the entering temperature between 300 K and 400K. Tubular (PFR) reactor volumes between 0.1 m3and 2 m3 are available for use.
- Determine the entering conditions of temperature and pressure and reactor volume that will optimize the production of methanol. (Hint: First try T0 = 330 at P0 = 40 atm, then try T0 = 380 and P0 = 1 atm)
- Vary the ratios of the entering reactants to CO to maximize methanol production. How do your results compare with those in part (A)? Describe what you find.
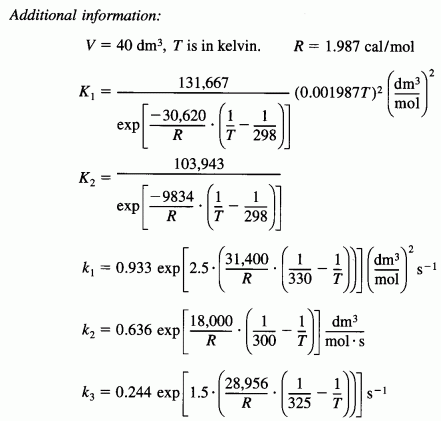
[3rd Ed. P6-24]