Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-EB
A mixture of 50% A, 50% B is charged to a constant-volume batch reactor in which equilibrium is rapidly achieved. The initial total concentration is 3.0 mol/dm3.
- Calculate the equilibrium concentrations and conversion of A at 330 K for the reaction sequence
Reaction 1: A + B
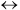 C + D Kc1(330 K)=4.0, Kc1(350 K)=2.63
C + D Kc1(330 K)=4.0, Kc1(350 K)=2.63Reaction 2: C + B
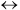 X + Y Kc2(330 K)=1.0, Kc2(350 K)=1.51
X + Y Kc2(330 K)=1.0, Kc2(350 K)=1.51 - Suppose now the temperature is increased to 350 K. As a result, a third reaction must now be considered in addition to the reactions above:
Reaction 3: A + X
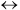 Z Kc3(350 K)=5.0 dm3/mol
Z Kc3(350 K)=5.0 dm3/molCalculate the equilibrium concentrations, conversion of A and overall selectivities
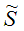 CX,
CX, 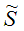 DZ, and
DZ, and 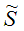 YZ.
YZ.
- Vary the temperature over the range 300 to 500 K to learn the effect of selectivities
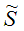 CX,
CX, 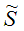 CZ, and
CZ, and 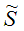 YZ on temperature.
YZ on temperature.
![]() HR1=
-20,000 J/mol A
HR1=
-20,000 J/mol A ![]() HR2= +20,000 J/mol B
HR2= +20,000 J/mol B
![]() HR3
-40,000 J/mol A
HR3
-40,000 J/mol A