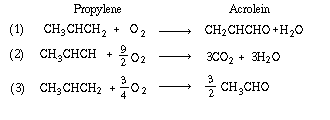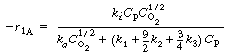Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-JC
| The oxidation of propylene (P) to acrolein (A) is carried out in a fluidized-bed reactor. The reactions taking place are [Chem. Eng. Sci., 51, 2189 (1996)]: | |||
|
|
|||
| Air and acrolein are fed to the reactor. The rate laws for the reaction (i= 1, 2, 3) are | |||
|
|
|||
| k i(appropriate units) | k a |
k1 |
k2 |
k3 |
| Frequency factor | 4 x 10 4 | 688 | 3.5 x 10 -4 | 32.8 |
| Activation energy (kJ/mol) | 88,000 | 77.500 | 1312 | 72,630 |
| The reaction is carried out isothermally. Plot the selectivity of
acrolein as a function of temperature and feed composition.
|