Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-ND
(Flame retardants) We now reconsider a more comprehensive version of the combustion of CO. The reactions and their corresponding rate law parameters are given in the table below. All reactions are assumed to be elementary [Combustion and Flame, 69 113 (1987)]. The precombustion compositions (mol %) are:
Without HCl: 20% CO 10% O2 1% H2 69 % N2
With HCl: 20% CO 10% O2 1% H2 67 % N2 2% HCl
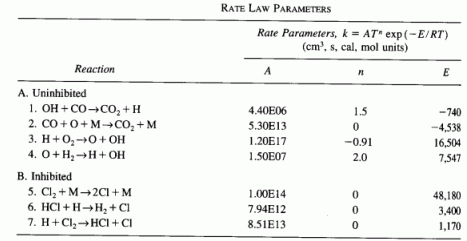
- Plot the mole fraction of each species and free radicals as a function of time for both uninhibited and inhibited combustion.
- Repeat part (A) for different temperatures and pressures.
(S. Senkin, UCLA)
[3rd Ed. P6-21]