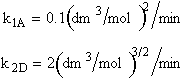Chapter 8: Multiple Reactions
Writing Net Rates of Formation
The reactions:
are elementary. Write the net rates of formation for A, B, C and D.
Hint 1: What is the net rate of formation of A?
$ (a) r_{A} = k_{1A}C_{A}{C_{B}}^2$
$ (b) r_{A} = -k_{1A}C_{A}{C_{B}}^2$
Hint 2: What is the net rate of formation of B?
$ (a) r_{B} = -k_{1A}C_{A}{C_{B}}^2-k_{2D}{C_{c}}^2{C_{B}}^{\frac{1}{2}}$
$ (b) r_{B} = -k_{1A}C_{A}{C_{B}}^2-6k_{2D}{C_{c}}^2{C_{B}}^{\frac{1}{2}}$
$ (c) r_{B} = -k_{1A}C_{A}{C_{B}}^2-\frac{1}{6}k_{2D}{C_{c}}^2{C_{B}}^{\frac{1}{2}}$
Hint 3: What is the net rate of formation of C?
$ (a) r_{C} = 2k_{1A}C_{A}{C_{B}}^2-\frac{2}{3}k_{2D}{C_{c}}^2{C_{B}}^{\frac{1}{2}} $
$ (b) r_{C} = 2k_{1A}C_{A}{C_{B}}^2-\frac{3}{2}k_{2D}{C_{c}}^2{C_{B}}^{\frac{1}{2}} $
$ (c) r_{C} = k_{1A}C_{A}{C_{B}}^2-\frac{2}{3}k_{2D}{C_{c}}^2{C_{B}}^{\frac{1}{2}} $
Hint 1
A.
![]()
![]()
![]()
Hint 2
B.
![]()

![]()
![]()
Hint 3
C.
![]()
![]()
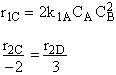
![]()
![]()
The reactions:
are elementary. Write the net rates of formation for A, B, C and D.
Solution
A.
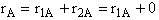
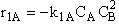
B.
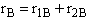

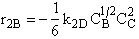
C.
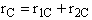
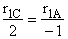
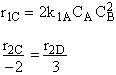
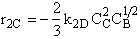
D.
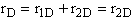
These net rates of reaction are now coupled with the appropriate mole balance of A, B, C, and D and solved using a numerical software package.
For example for a PFR:
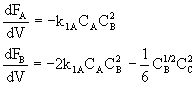
etc.