Chapter 9: Reaction Mechanisms, Pathways, Bioreactions and Bioreactors
Additional Homework Problems
CDP9-KB
The instantaneous number-average degree of polymerization, XN, can be expressed as
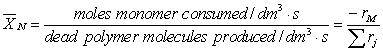 (CDP7-N1)
(CDP7-N1)
(a) Consider an additional termination step resulting from the combination of the initiator molecule and a free radical Rj:
![]() (CDP7-N2)
(CDP7-N2)
(b) Show that
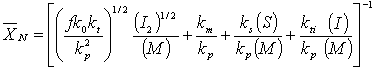 (CDP7-N3)
(CDP7-N3)
where
![]()
(c) Neglecting solvent transfer, show that
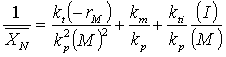
(d) Explain how you can determine rate law parameters from experimental data obtained in a CSTR. Use sketches to elucidate your explanation.
(e) Typical activation energies for the initiation, propagation, and termination steps are: 20 kJ/mol, 20 kJ/mol, and 9 kJ/mol. discuss the effect of temperature on free-radical polymerization.