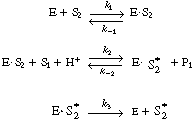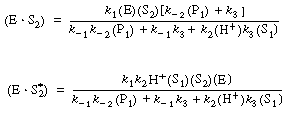Chapter 9: Reaction Mechanisms, Pathways, Bioreactions and Bioreactors
Professional Reference Shelf
Example R9.6-2: Derive an Initial Rate Law for Alcohol Dehydrogenates
|
Before considering the possibility of going directly from the apoenzyme to the holoenzyme, assume that the
rate of dissociation of the complex (ADH |
|||
|
|
|||
is irreversible, and show that the initial rate law for ethanol in the enzyme cofactor reaction sequence discussed earlier is of the form |
|||
|
|
(RE7.4-2.1) |
||
| |
|||
| Solution | |||
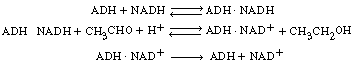 |
|||
Let E = ADH, S1 = CH3CHO, S2 = NADH, |
|||
|
|
|||
By adding the rate law for the rate of formation of ethanol (P 1), |
|||
|
|
|||
to the equation for |
|||
|
|
(RE7.4-2.2) |
||
Application of the PSSH to the holoenzyme (E |
|||
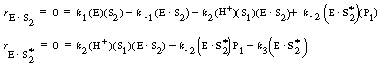 |
|||
allows one to solve for the concentrations of the cofactor-enzyme complexes in terms of S2, S2*, and E. |
|||
|
|
(RE7.4-2.3) |
The total concentration of bound and unbound enzyme is |
|||
|
|
(RE7.4-2.5) |
||
Substituting Equations (RE7.4-2.3) and (RE7.4-2.4) into Equation (RE7.4-2.5), the unbound enzyme concentration is |
|||
(RE7.4-2.6) |
|||
Setting (P1) = 0, we obtain the initial rate law by combining Equations (RE7.4-2.2), (RE7.4-2.4), and (RE7.4-2.6): |
|||
|
|
(RE7.4-2.7) |
||