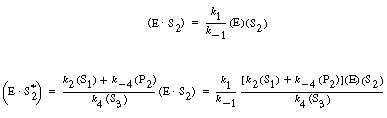Chapter 9: Reaction Mechanisms, Pathways, Bioreactions and Bioreactors
Professional Reference Shelf
Example R9.6-3: Derive a Rate Law for a Multiple Substrate System
|
Derive the rate law for this sequence assuming that the rate of reaction
between the (ADH |
|||
| Solution | |||
| Assuming that the reaction between the cofactor-enzyme complex and acetaldehyde is rate limiting, the rate law is | |||
|
|
|||
| [Note: k2=k2
(h+).] After applying the PSSH
to (E (E |
|||
|
|
(RE7.4-3.1) (RE7.4-3.2) |
||
| we add Equations (RE7.4-3.1) and (RE7.4-3.2) and rearrange to obtain | |||
|
|
(RE7.4-3.3) (RE7.4-3.4) | ||
| The total enzyme concentration is again constant at (Et): | |||
|
|
(RE7.4-3.5) (RE7.4-3.6) | ||
| After solving Equation (RE7.4-3.6) for E, we combine with Equation (RE7.4-3.1) to obtain the rate law: | |||
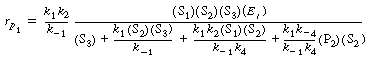
|
(RE7.4-3.7) | ||
| Setting P2 = 0 and rearranging, we obtain the initial rate law: | |||
|
|
(RE7.4-3.8) | ||
| where | |||
|
|