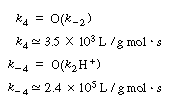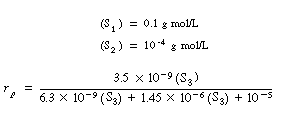Chapter 9: Reaction Mechanisms, Pathways, Bioreactions and Bioreactors
Professional Reference Shelf
Example R9.6-4: Calculate the Initial Rate of Formation of Ethanol in the Presence of Porpanediol
|
Assume that the specific reaction rates for the conversion of propanediol
to DL -lactaldehyde are the same order of magnitude
as the corresponding specific reaction rates for the conversion of
ethanol to acetaldehyde. Calculate the initial rate of formation of ethanol
in the presence of propanediol and compare this rate with the initial rate
when propanediol is absent from the system. Solution Assuming the corresponding specific reaction rates to be equal, |
|||
|
|
|||
| We can then obtain numerical values for Vmax, K3, and K3 in Equation (RE7.4-3.8): | |||
|
Vmax = (k4)(Et) = (3.5 X 103)(10-7) = 3.5 x 10-4S-1 |
|||
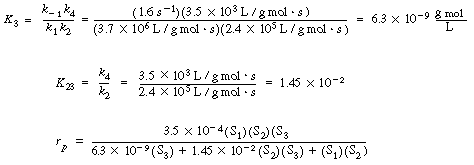
initially
| ||
If
|
||
we can neglect the first two terms in the denominator with respect to the last term, |
||
|
|
||
When no propanediol was present, we found that |
||
|
|
||
If the propanediol and acetaldehyde are present in the same initial concentration, |
||
|
|
||
then |
||
|
|
||
and we see that the addition of the third substrate increases the rate of reaction by a factor of 10! |