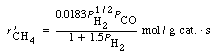Chapter 10: Catalysis and Catalytic Reactors
Additional Homework Problems
CDP10-GB
| The use of a differential reactor to study the formation of methane from hydrogen and carbon monoxide over a nickel catalyst gave the following: | ||
|
|
||
| Suggest a mechanism and rate-limiting step that is consistent with the experimental observation. | ||
|
It is desired to produce 20 tons/day of CH 4 . Calculate the catalyst weights necessary to achieve 80% conversion in: (a) A fixed bed. The feed consists of 75% H 2 and 25% CO at a temperature of 500°F and a pressure of 10 atm. Assume both molecular and atomic hydrogen are adsorbed on the surface. |