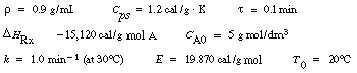Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Additional Homework Problems
CDP12-ID
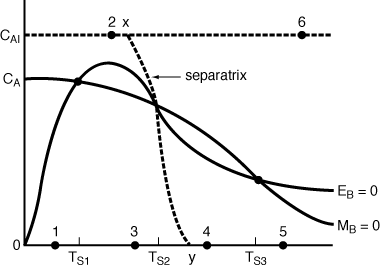
| The concentration-temperature curves for steady energy and mole balances are shown in Figure CDP12-I for a particular reaction. Sketch the various approaches to steady state starting at the different initial conditions shown for points 1 through 6. (Hint: See Figure 7.18, R. Aris, Elementary Reactor Analysis (Upper Saddle River, N.J.: Prentice Hall, 1969).] | ||
|
||
| Next consider a first-order irreversible exothermic liquid-phase reaction |
||
|
|
||
|
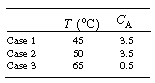
The following questions concern variation in one parameter with the others fixed at the values given. (a) At what space-times do ignition and extinction (blowout)
occur? |
||
|
|
||
| (R. L. Curl, University of Michigan) [2nd Ed. P8-22] |