Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Derivation
The governing equations for a PFR/PBR:
Mole Balance:
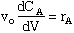
Energy Balance:

Solutions to these equations give the following typical profiles (in the absence of runaway):

Figure 5. Temperature and concentration profiles.
At the maximum temperature in the reactor, Tm

then

For a first order reaction:
, then
and we can solve for CAm to obtain

where Tm is the maximum temperature in the reactor and CAm is the corresponding concentration of A at that maximum temperature. For this reactor
.