Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Example - Using N/S vs. S Plot
The elementary gas phase reaction
![]()
is to be carried out in a PFR with external cooling. The entering pressure is 24.6 atm and the entering temperature is 227°C. The feed is 66.7% A and 33.3% inert. The ambient temperature in the heat exchange is constant at 500K. What is the largest tube diameter one can have and not have runaway.
Additional Information


Hint 1 Calculate S

at T = T0

![]()
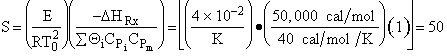

Hint 2 - Calculate N



![]()
![]()

![]()
Hint 3 - Find N/S for = 50
For S = 50 and a 2nd order reaction (n=2) We find from Figure on the web ![]() = 1.6
= 1.6
Hint 4 - Calculate dt
![]()
![]()
One could have calculated the ratio.
One could have calculated the ratio.
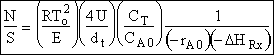
without calculating N separately.