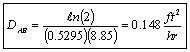Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Learning Resources
Example CD14-4: Measuring Gas Phase Diffusivities
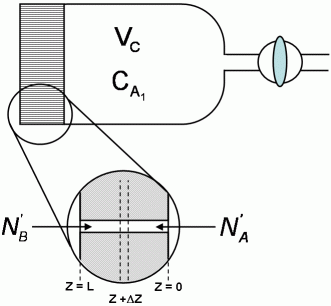
A diffusion cell is used to measure binary molecular diffusivities. It consists of a chamber closed at one end by a fine porous glass disc.
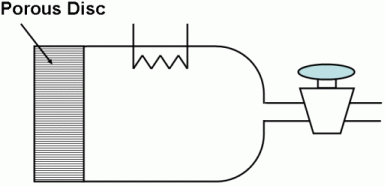
In use, the chamber is filled through the stopcock with a pure gas, the stopcock is closed, and the cell is immersed in the other gas. Analysis of the gas in the cell is obtained continuously by an internal thermal conductivity device.
The cell has been calibrated with hydrogen-air at 0 and 1 atm. DAB = 2.37 ft2/hr by noting the time it took for the internal concentration to fall to 20
and 1 atm. DAB = 2.37 ft2/hr by noting the time it took for the internal concentration to fall to 20 of its original value was 1 hour and 17 minutes.
of its original value was 1 hour and 17 minutes.
The molecular diffusivity of phosgene is measured similarly at 20 and 1 atm.
It is found that the concentration reaches half of its original concentration in 8 hours and 51 minutes.
and 1 atm.
It is found that the concentration reaches half of its original concentration in 8 hours and 51 minutes.
What is the molecular diffusivity of phosgene at 20 ?
?
State all of your assumptions.
Solution
For equi-molar counter diffusion:
A mass balance on the element 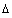 z of an assumed set of parallel cylindrical pores gives:
z of an assumed set of parallel cylindrical pores gives:
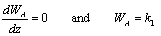
Applying Fick's Law with (WA + WB) = 0 gives:
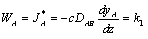
Rearranging and integrating:
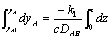
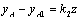
assuming yA = 0 when z = L
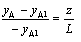
evaluating 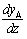 from the above and substituting into Fick's Law
from the above and substituting into Fick's Law
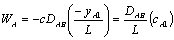
Assuming quasi-stead state, a mass balance on the diffusion cell gives:

Substituting for WA and rearranging with vc constant
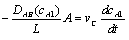
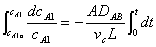

evaluating the cell constant, k2, from data in case 1 gives:
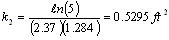
for the phosgene-air system, (case 2)