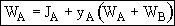Chapter 14: External Diffusion Effects on Heteregeneous Reactions

Where VA is the particle velocity of A which is the vector average of millions of A molecules at a point. VA is the velocity wrt to a stationary coordinate, e.g., the lab bench.

where V is the molar average velocity of all species

multiplying by the total concentration.
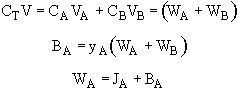
JA tells us of the flux of A with respect to the molar average velocity.